The skin is a barrier organ that is exposed to a wide variety of potential pathogens including bacteria, fungi and viruses. Within the skin there are numerous components of both the innate and adaptive immune system.
The Kaplan Lab is focused on understanding the cellular immune circuits that define responses to infection or damage and maintain immune homeostasis in peripheral tissue with an emphasis on the skin. Areas of focus include how sensory neurons that trigger pain or itch sensation directly modulate innate and adaptive immune responses in the perturbed state and alter the inflammatory ‘set-point’ in the skin during homeostasis. We are also interested in the formation of resident memory T cells and how tissue-specific factors can determine the fitness of these cells. Our approach employs genetic gain- and loss-of-function murine models with inflammatory or infectious triggers which we combine functional, transcriptomic and spatial techniques to define the response. Key findings are confirmed in human tissues where possible.
There are several major projects in lab:
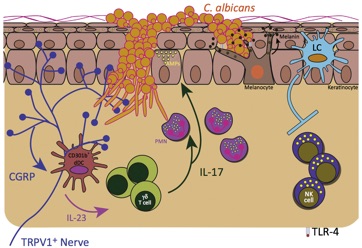
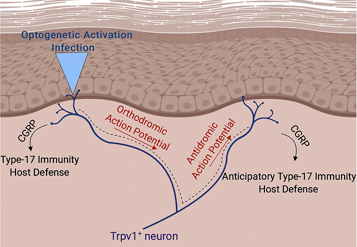
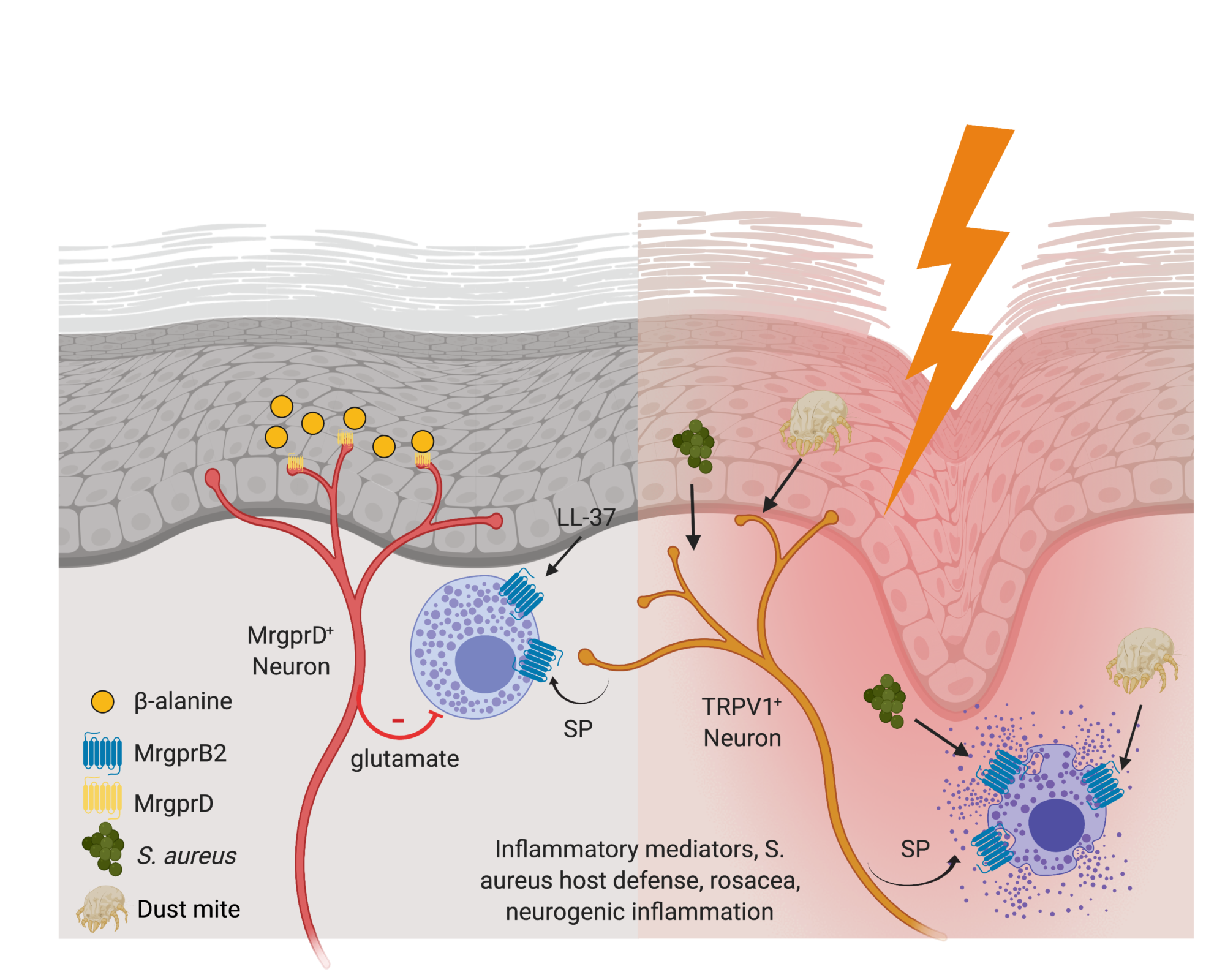
Control of Cutaneous Immunity by Sensory Afferent Nerves
We are using a combination of optogenetics and deletional strategies to define the precise interaction between subsets of afferent fibers and individual components of the adaptive and innate skin immune system.
Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity. 2015 Sep 15;43(3):515–26. With a preview by Rich Gallo.
Kashem SW, Kaplan DH. Skin Immunity to Candida albicans. Trends Immunol. 2016 May 10;37(7):440–50.
Kaplan DH. Ontogeny and function of murine epidermal Langerhans cells. Nat Immunol. Nature Publishing Group; 2017 Sep 19;18(10):1068–75.
Cohen JA, Edwards TN, Liu AW, Hirai T, Ritter-Jones M, Li Y, Zhang S, Ho, J, Davis BM, Albers KM, Kaplan DH. Cutaneous TRPV1+ neurons trigger protective Innate Type-17 anticipatory immunity. 2019 Cell. With a preview by Brian Kim.
Shiqun Zhang , Tara N Edwards , Virendra K Chaudhri , Jianing Wu, Jonathan A Cohen, Toshiro Hirai , Natalie Rittenhouse , Elizabeth G Schmitz , Paul Yifan Zhou , Benjamin D McNeil , Yi Yang, H Richard Koerber , Tina L Sumpter , Amanda C Poholek, Brian M Davis, Kathryn M Albers, Harinder Singh, Daniel H Kaplan. Nonpeptidergic neurons suppress mast cells via glutamate to maintain skin homeostasis. 2021 Cell.
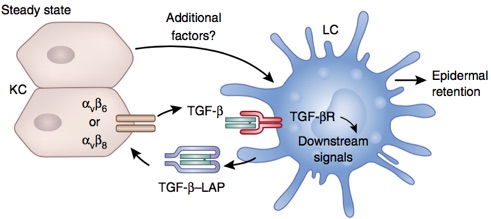
Langerhans cell and Resident memory T cell epidermal residency is maintained by Keratinocyte activation of TGFβ
We are currently exploring the how integrin-mediated activation of TGFβ is controlled. We believe that keratinocytes control of integrin expression determines whether LC and TRM persist in the epidermis. This has important implications for the development of local memory responses and autoimmune disease. Our approach centers around in vivo manipulation of integrin expression and sensitivity to TGFβ by LC and T cells.
Bobr A, Igyarto BZ, Haley KM, Li MO, Flavell RA, Kaplan DH. Autocrine/paracrine TGF-β1 inhibits Langerhans cell migration. Proc Natl Acad Sci USA. National Acad Sciences; 2012 Jun 26;109(26):10492–7.
Mohammed J, Beura LK, Bobr A, Astry B, Chicoine B, Kashem SW, et al. Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-β. Nat Immunol. Nature Research; 2016 Apr;17(4):414–21.
Kashem SW, Haniffa M, Kaplan DH. Antigen-Presenting Cells in the Skin. Annu Rev Immunol. Annual Reviews; 2017 Apr 26;35(1):469–99.
Kaplan DH. Ontogeny and function of murine epidermal Langerhans cells. Nat Immunol. Nature Publishing Group; 2017 Sep 19;18(10):1068–75.
Hirai T, Zenke Y, Yang Y, Bartholin L, Beura LK, Masopust D, et al. (2019). Keratinocyte-Mediated Activation of the Cytokine TGF-β Maintains Skin Recirculating Memory CD8+ T Cells. Immunity, 50(5), 1249–1261.e5. PMCID: PMC6531326
Hirai, T., Yang, Y., Zenke, Y., Li, H., Chaudhri, V.K., Diaz, J.S.D.L.C., Zhou, P.Y., Nguyen, B.A.-T., Bartholin, L., Workman, C.J., et al. (2021). Competition for Active TGFβ Cytokine Allows for Selective Retention of Antigen-Specific Tissue- Resident Memory T Cells in the Epidermal Niche. Immunity 54, 84-98.e5. With a preview by John Chang.
Whitley, S. K., M. Li, S. W. Kashem, T. Hirai, B. Z. Igyártó, K. Knizner, J. Ho, L. K. Ferris, C. T. Weaver, D. J. Cua, M. J. McGeachy, and D. H. Kaplan. 2022. Local IL-23 is required for proliferation and retention of skin-resident memory TH17 cells. Sci Immunol 7: eabq3254
Legacy Projects
Role of individual DC subsets in defining adaptive responses to cutaneous antigen
Our current effort on this project focuses on the requirement for local DC produced cytokines to promote and maintain resident memory T cell populations in the setting of infection and autoimmunity.
Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011 Aug 26;35(2):260–72.
Haley K, Igyarto BZ, Ortner D, Bobr A, Kashem S, Schenten D, et al. Langerhans cells require MyD88-dependent signals for Candida albicans response but not for contact hypersensitivity or migration. J Immunol. 2012 May 1;188(9):4334–9.
Kashem SW, Igyarto BZ, Gerami-Nejad M, Kumamoto Y, Mohammed J, Jarrett E, et al. Candida albicans Morphology and Dendritic Cell Subsets Determine T Helper Cell Differentiation. Immunity. 2015 Feb 17;42(2):356–66.
Kashem SW, Haniffa M, Kaplan DH. Antigen-Presenting Cells in the Skin. Annu Rev Immunol. Annual Reviews; 2017 Apr 26;35(1):469–99.
Langerhans Cells promote humoral responses
Yao C, Kaplan DH. Langerhans Cells Transfer Targeted Antigen to Dermal Dendritic Cells and Acquire Major Histocompatibility Complex II In Vivo. J Invest Dermatol. 2018 Feb 21.
Yao C, Zurawski SM, Jarrett ES, Chicoine B, Crabtree J, Peterson EJ, et al. Skin dendritic cells induce follicular helper T cells and protective humoral immune responses. J Allergy Clin Immunol. 2015 Nov;136(5):1387–97.e1–7.